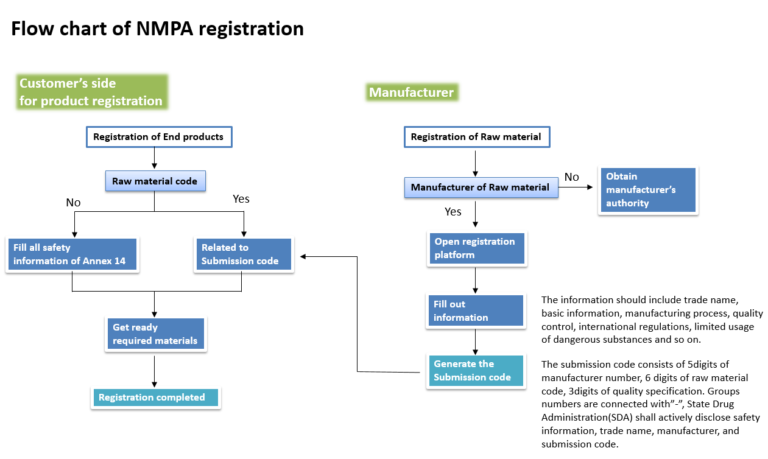
Nmpa China New Submissions Guideline. Registrants are required to prepare the report annually for all medical devices (including IVDs) listed in China. The Draft Amendment, once approved and released by the State Council, will significantly impact the operations of pharmaceutical companies. The format and arrangement of content stipulated are now part of the regulatory requirements to file for NMPA submission. The PDF submission document must be readable and searchable under the new guidelines. The drug category in which an applicant chooses to register determines the clinical trial application review and approval or filing process. Electronic Regulated Product Submission, the NMPA's new registration system.

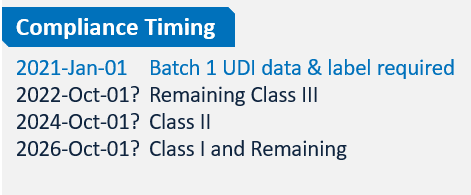
Nmpa China New Submissions Guideline. The NMPA is responsible for the registration of medical devices for the Chinese market. The Draft Amendment, once approved and released by the State Council, will significantly impact the operations of pharmaceutical companies. Registrants are required to prepare the report annually for all medical devices (including IVDs) listed in China. An electronic signature is applied and validated. The PDF submission document must be readable and searchable under the new guidelines. All medical devices have to be classified by the NMPA according to its risk in three classes. Nmpa China New Submissions Guideline.
For background on China's reformation of the review and approval system to encourage innovation of drugs, see the SC.
The Draft Amendment also reflected the NMPA's new attempt to address issues of greater public concern, such as penalty guidelines for violations of the DAL.
Nmpa China New Submissions Guideline. Renewal of medical device products will require a new report submission. This new guideline highlights the language requirement for the submission components, including study database, analysis database, data definition file, data reviewer's guide and annotated CRF. Innovation in manufacturing techniques has encouraged China to explore the scope of research and development for medicinal preparations. Registrants are required to prepare the report annually for all medical devices (including IVDs) listed in China. Electronic Regulated Product Submission, the NMPA's new registration system. This included updates to requirements for the filing and binding review of APIs, excipients, and pharmaceutical packaging materials.
Nmpa China New Submissions Guideline.