Nmpa China Guidelines. The standards cover indications of orthopedic, cardiovascular. The revised or newly established guidelines are aimed to facilitate manufacturers with local type testing, pre-clinical, clinical trial, and regulatory submission, and make guidelines more consistent with the international counterparts. China will roll out a raft of stronger supportive policies to boost the development of the pharmaceutical and medical equipment industries, as part of the country's efforts to strengthen areas of weakness and enhance market competitiveness, the State Council, or China's Cabinet, said on Friday. The guideline applies to invasive CGMS and excludes non-invasive and implantable CGMS. These were put in place to guide manufacturers for local type testing, clinical trials, and regulatory approval. The revisions are aimed to facilitate manufacturers with local type testing and make guidelines more consistent with the international counterparts.

Nmpa China Guidelines. China's National Medical Products Administration ("NMPA") recently announced a comprehensive draft amendment ("Draft Amendment") to the Implementation Regulation of the PRC Drug Administration Law ("DAL Implementing Regulations"). Full List Clinical evaluation guideline on predicate comparison of positron emission/X-ray computed tomography system nmpa.gov.cn. Overseas inspection is the global common way for medical device inspection and one of the important steps for Chinese medical device inspection to be internationalized. The Standards Revisions are aimed to facilitate manufacturers with local type testing and regulatory submission and make standards more consistent with the. The standards cover indications of orthopedic, cardiovascular. The revisions are aimed to facilitate manufacturers with local type testing and make guidelines more consistent with the international counterparts. Nmpa China Guidelines.
The newly developed antibacterial sutures are coated with triclosan, a broad-spectrum antimicrobial agent.
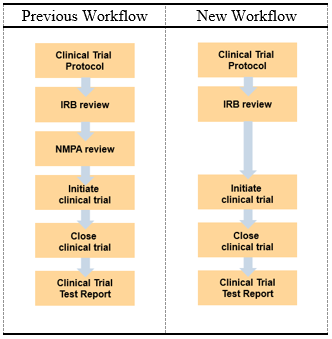
These were put in place to guide manufacturers for local type testing, clinical trials, and regulatory approval.
Nmpa China Guidelines. Guidelines & Standards How is CGMS Approved? China classifies medical devices including in vitro diagnostic reagents into three categories according to risk levels. The guideline applies to invasive CGMS and excludes non-invasive and implantable CGMS. The standards cover indications of orthopedic, cardiovascular. The DRR clarifies that the NMPA regulates clinical trials for drugs in development that are ultimately. The revisions are aimed to facilitate manufacturers with local type testing and make guidelines more consistent with the international counterparts.
Nmpa China Guidelines.