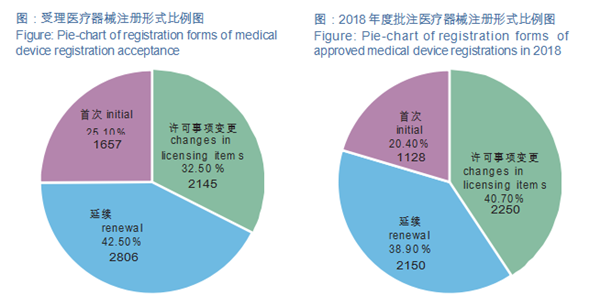
Nmpa China Medical Device Regulations. The Standards Revisions are aimed to facilitate manufacturers with local type testing and regulatory submission and make standards more. Under the China medical device regulations, devices can be categorized into Class I, Class II, and Class III devices. The NMPA was also known as China Food and Drug Administration in the past. Our specialists work directly with the NMPA on a daily basis, coordinating the submission of regulatory documents and answering NMPA follow-up questions. Determine if clinical trials will be needed for your device. The revisions are aimed to facilitate manufacturers with local type testing and make guidelines more consistent with the international counterparts.

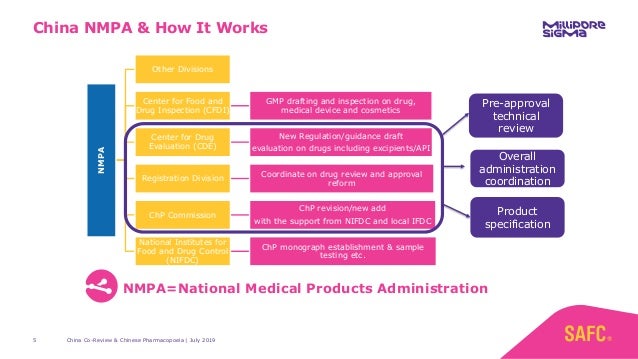
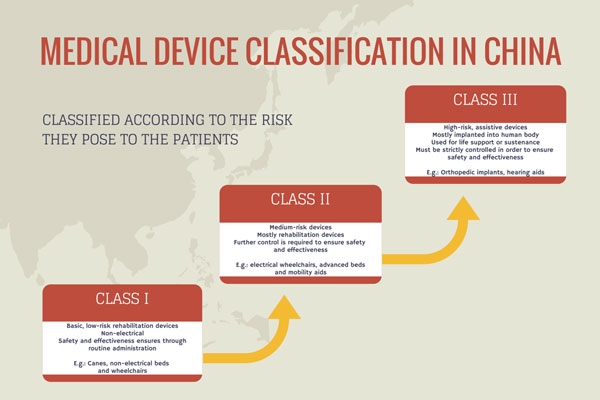
Nmpa China Medical Device Regulations. For medical device manufacturers, understanding these regulations is essential. China National Medical Products Administration regulates medical devices and pharmaceutical products across China. Under the China medical device regulations, devices can be categorized into Class I, Class II, and Class III devices. These were put in place to guide manufacturers for local type testing, clinical trials, and regulatory approval. The results of the reform of "decentralization, regulation and service" encourage the innovation and development of the industry and. With the NMPA approval, the platform can now be used in clinical applications for DNA and RNA sequencing in China, "further augmenting the company's competitiveness," according to. Nmpa China Medical Device Regulations.
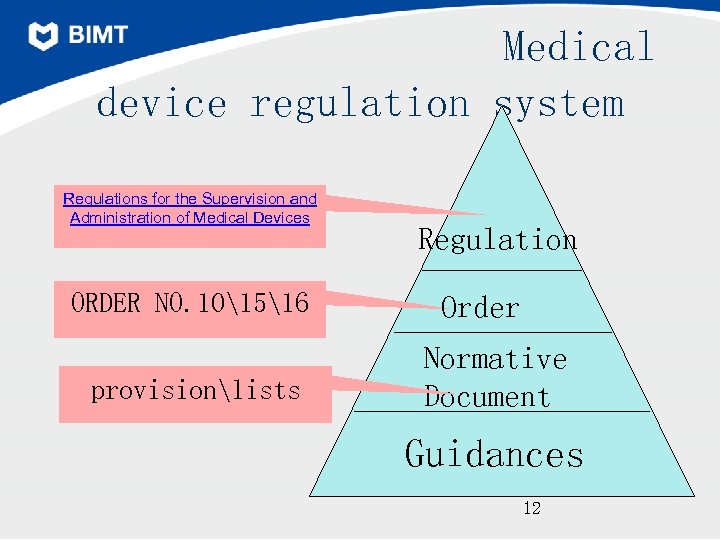
Since then diverse provisions, notifications and technical guidances are published which should be comply with and followed in the.
Under the China medical device regulations, devices can be categorized into Class I, Class II, and Class III devices.
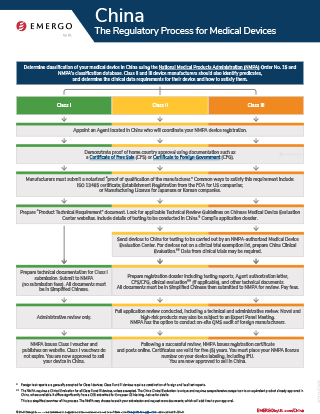
Nmpa China Medical Device Regulations. The China medical device quality and safety. The Standards Revisions are aimed to facilitate manufacturers with local type testing and regulatory submission and make. The results of the reform of "decentralization, regulation and service" encourage the innovation and development of the industry and. Emergo can help you obtain regulatory approval for your medical device or IVD in China. Chinese NMPA Regulatory Approval Process for Medical and IVD Devices To see their products in the Chinese market, medical and in vitro diagnostic (IVD) device manufacturers will need to obtain National Medical Products Administration (NMPA) approval. These sutures are coated with a triclosan antimicrobial agent that exhibits activity against.
Nmpa China Medical Device Regulations.