Nmpa China Cosmetics. New cosmetic ingredients may need to consider new cosmetic ingredient filing/registration in China and/or China REACH. NMPA Announcement on the Good Manufacturing Practice for Cosmetics. In China, the Cosmetics Supervision and Administration Regulation (CSAR) requires submission of safety assessment report for cosmetic products and new cosmetic ingredients for notification or registration purposes. China NMPA Publishes Official Q&A for New Cosmetics Registration and Notification Policy. Here are the highlights in this new provision. Section I Registration and Filing of New Cosmetic Ingredients.

Nmpa China Cosmetics. Drugs, APIs, Pharmaceuticals excipients, pharmaceutical packaging materials, medical devices, diagnostic reagents, cosmetics, etc. FAQs from NMPA on China Cosmetic Supervision and Administration. With the NMPA approval, the platform can now be used in clinical applications for DNA and RNA sequencing in China, "further augmenting the company's competitiveness," according to a company filing with the Shanghai Stock Exchange last Friday. The exact costs and the timeframe depend, among other things, on the type of cosmetics, the ingredients and the tests to be carried out in China. Section I Registration and Filing of New Cosmetic Ingredients. Cosmetic products with efficacy claims shall be supported by an efficacy evaluation. Nmpa China Cosmetics.
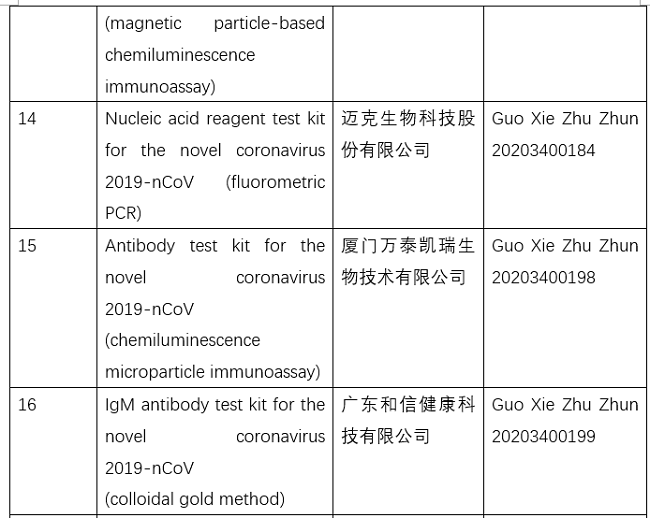
NMPA Related Administrative Licensing Items: Project Code.
The exact costs and the timeframe depend, among other things, on the type of cosmetics, the ingredients and the tests to be carried out in China.
Nmpa China Cosmetics. NMPA Announcement on the Good Manufacturing Practice for Cosmetics. China cosmetics quality and safety responsibilities regulations have been released by the National Medical Products Administration (NMPA). Cosmetic products with efficacy claims shall be supported by an efficacy evaluation. Section I Registration and Filing of New Cosmetic Ingredients. Here, cosmetic ingredient manufacturers or authorized enterprises can log on to submit ingredient safety-related information, according to ChemLinked. In order to implement the Provisions for the Registration and Filing of Cosmetics, Provisions for the Registration or Filing Dossier of Cosmetics and other regulatory documents, NMPA organized to establish the Cosmetic Ingredient Safety Information Registration Platform.
Nmpa China Cosmetics.