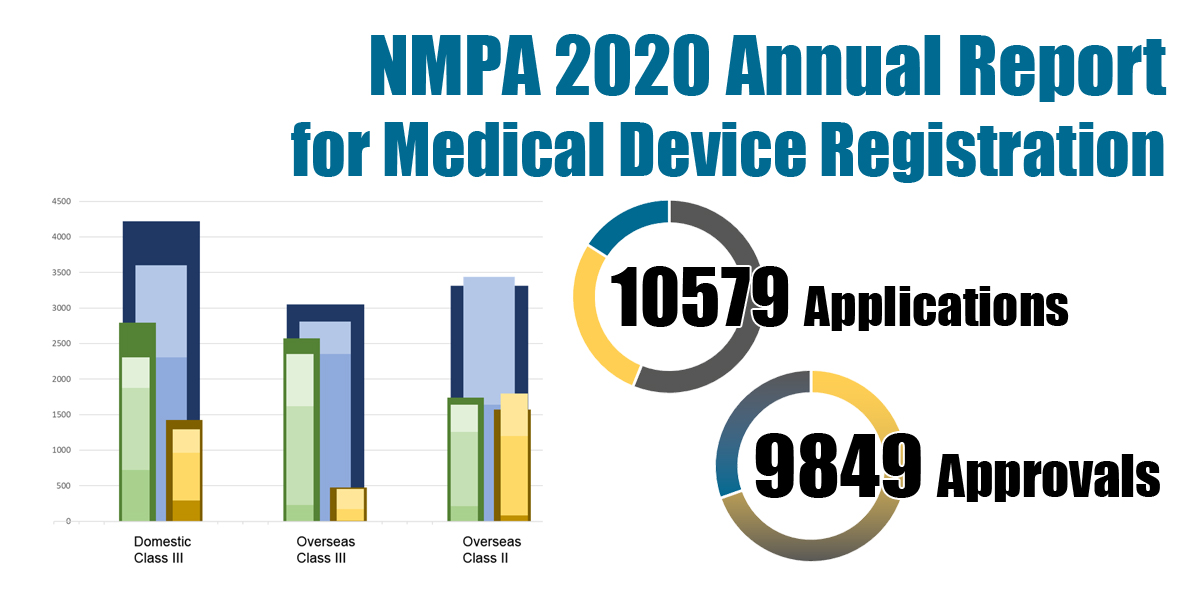
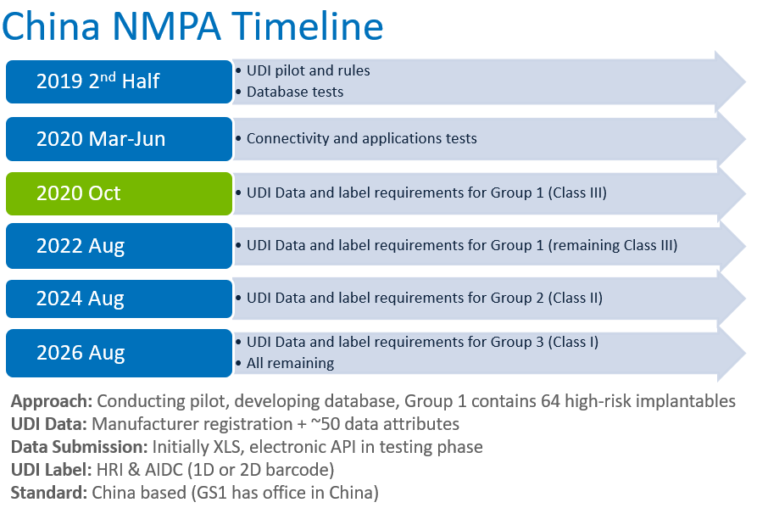
Nmpa China. Policies More Class II & III Medical Devices Exempted from Clinical Trial NMPA is responsible for conducting drug registration and approvals, provides guidance to provincial authorities and works with provincial level investigators to assign inspections of clinical. Recently, the innovative product Coronary Artery CT Fractional Flow Reserve Calculation Software of Shanghai Pulse Medical Technology Co., Ltd. is approved by China NMPA. List of Approved Domestic Vaccine Products in China. These updates are presented by China Med Device, LLC, your partner in Chinese market access. Depending on the risk classification, different aspects are required: For medical devices of class I: product tests are sometimes required Database. In China, Pharmaceuticals are regulated by The National Medical Products Administration (NMPA) is the Chinese agency for regulating drugs and medical devices (formerly known as the China Food and Drug Administration or CFDA).

Nmpa China. China's National Medical Products Administration (NMPA) has given the green light to Gloria Biosciences Co. NMPA is listed in the World's most authoritative dictionary of abbreviations and acronyms. The NMPA requires comprehensive documentation, and all the documents must be submitted in the original language, if applicable and as well as in Chinese. The newly developed antibacterial sutures are coated with triclosan, a broad-spectrum antimicrobial agent. Policies More Class II & III Medical Devices Exempted from Clinical Trial NMPA is responsible for conducting drug registration and approvals, provides guidance to provincial authorities and works with provincial level investigators to assign inspections of clinical. Recently, the innovative product Coronary Artery CT Fractional Flow Reserve Calculation Software of Shanghai Pulse Medical Technology Co., Ltd. is approved by China NMPA. Nmpa China.
Laws & Regulations The NMPA is responsible for registration of medical devices for the Chinese market.
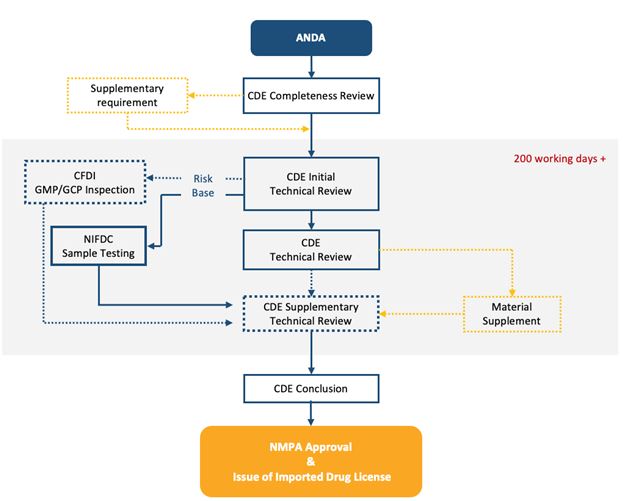
The drug category in which an applicant chooses to register determines the clinical trial application review and approval or filing process.
Nmpa China. All content on this website, including dictionary, thesaurus, literature, geography, and other reference data is for informational purposes only. Recently, the innovative product Coronary Artery CT Fractional Flow Reserve Calculation Software of Shanghai Pulse Medical Technology Co., Ltd. is approved by China NMPA. All medical devices have to be classified by the CFDA according to its risk in three classes. NMPA – What does NMPA stand for? New guidance regarding the Phase III pre-clinical trial meeting with the Center for Drug Evaluation when researching innovative drugs (See Submission Process); New guidelines related to the summary, analysis, and reporting of safety. These updates are presented by China Med Device, LLC, your partner in Chinese market access.
Nmpa China.